A Write true or false for each statement. Water has a mass per mole of 180 gmol and each water molecule H2O has 10 electrons.

Water Has A Mass Per Mole Of 18 0 G Mol And Each Water Molecule H2o Has 10 Homeworklib
Answers are on the answer page as usual.

. And is a mole of water equal to 180 g. NH4NO3 N2O 2H2O. How many molecules of water have a mass of 54 g.
B What is the net charge of all these electrons. Amount of moles in the sample. What is the mass in grams of one mole of 12 c.
A A mole of horses contain a mole ot horse legs. Solution for Water has a mass per mole of 180 gmol and each water molecule H2O has 10 electrons. Given mass of water H₂O 18 grams.
Therefore knowing the molar mass the number of moles in a container can be calculated using the expression. M How many grams of water molar mass180 gmole must be added to 200 grams of CaCO 3 molar mass100 gmole to make an aqueous solution that has a mole fraction of solute of 0100. How many moles of water are contained in this sample.
So you found that in the previous from its 1000 grams and the molar mass water they tell you is or the Master mo is 18 grams per mole. Now since it is given that. Mass of the water 7740 g.
How many moles of water are contained in this sample. In the question given that Molar mass of water H₂O 18 gmole. A solution that is 756 by mass NaNO 3 molar mass850 gmole in water molar mass180 gmole has a density of 109 gmL.
Electrons are present is 774 L of water. B A mole of water has a mass of 180 g. The molar mass of substance is a property defined as its mass per unit quantity of substance in other words molar mass is the amount of mass that a substance contains in one mole.
In this case you know that the molar. Density of water 1000 gL. So that makes the second statement true.
A How many electrons are there in 5 liters of water. A sample of water has a mass of 180 g. A Write true or false for each bartleby.
N WM where. A chemist who is performing this reaction starts with 1601 g of NH4NO3. Now since it is given that molar mass of water is 180 grams this means that 1 mole of water contains 18 grams of water.
Number of water molecules in 430 moles. 643 g 1 mole180 g 357 mole. Moles of water molecules.
Water has a mass per mole of 180 gmol and eachj water molecule 68273 results. 𝑵𝑻𝒐 𝒂 𝑵 𝒐 𝒆 𝒆 𝒐 𝒆𝒄 𝒆𝑵𝑨. The molar mass of water.
Hence the no of molecules in n moles of water equals. A Write true or or each statement. C The mass of 1 molecule of water is 180 g.
I just multiplying the number of moles you found before. How many moles of hydrogen atoms is 45 x 1036 atoms. How many electrons are there in 3 liters of water.
Volume of water 774 L. The molar mass of NH4NO3 is 8003 gmol. A How many electrons are there in one liter 100 10-3m3 of water SolutionInn.
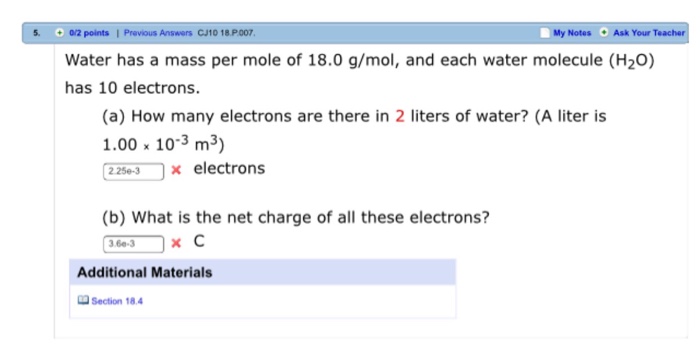
Thats just the mass of water. Water has a mass per mole of 180 gmol and each water molecule H2O has 10 electrons. No we just said the mass of a mole of water equals 18 g and a mole you might think is short for a.
A How many electrons are there in 5 liters of water. A How many electrons are there in 526 liters of water. Oxygen gas is O2 with a molar mass of 32.
Now by definition of mole we know that 1 mole of any substance is Avagadro Number of particles. D A mole of NaCis contains 2 moles of ions. How many grams of water H_2O have the same number of oxygen atoms as 10 mol of oxygen gas.
A Molar mass of the water 18 gmol. Moles of any substance will be calculated as. B How many carbon.
Water has a mass per mole of 180 gmol and each water molecule H 2 O has 10 electrons. A sample of water has a mass of 18. We get around 5555 moles and from there you can find a number of electrons.
M molar mass. Hence by ratio and proportion number of moles in 1 kg water equals. Now we put all these values in the above equation we get.
A How many electrons are there in one liter 100 x 10-3 m3 of water. 1 liter is equivalent to 100 10-3 m3 b What is the net charge of all these electrons. A A mole ofhorses contain a mole of horse legs.
Ready for a quiz. Moles of H₂O 18 grams 18 gmole 1 mole. 54 g 1 mole180 g 6022 x 1023 molecules 1 mole 18 x 1023 molecules.
Water has a mass per mole of 180 gmol and each water molecule H2O has 10 electrons. Mass of water in 774 L of water. How many roses do you have if you have 25 mole.
W given or required mass. The chemical equation below shows the decomposition of ammonium nitrate NH4NO3. 1 molecule water contains 10 electrons.
Because the molar mass of water which has two hydrogen Which has a massive one 1 oxygen which has a massive 16 has a mass of 18 g per mole. What is its molarity. The molar mass of water H2O is 18.
C The mass of 1 molecule of water is 180 g. Atomic mass of H is 1 and O is 16 so H2O has a molar mass of 18. Answer to Water has a mass per mole of 180 gmol and each water molecule H2O has 10 electrons.
Physics questions and answers. Now is the mass of one molecule 18 g. B A mole of water has amass of 180 g.
A liter is 100 10-3 m3 electrons. A solution that is 756 by mass NaNO3 molar mass850 suolo g mole in water molar mass180 gmole has a density of.

The Molar Mass Of Water Is 18 0 Grams Per Mole What Is The Mass Of 9 00 Moles Of Water Brainly Com
Solved Water Has A Mass Per Mole Of 18 0 G Mol And Each Chegg Com

Solved 2 Water Has A Mass Per Mole Of 18 0 G Mol And Each Chegg Com
0 Comments